2 Chemistry of Living Things
2.1 Introduction
As you read this chapter, you will learn more about how chemistry relates to our lives, health, and the foods we eat. Specifically, you will learn about:
• The nature of chemical substances, including elements, compounds, and their component atoms and molecules.
• The structures and functions of biochemical compounds, including carbohydrates, lipids, proteins, and nucleic acids (such as DNA and RNA).
• What chemical reactions are, how energy is involved in chemical reactions, how enzymes assist in chemical reactions, and some types of biochemical reactions in living organisms.
• Properties of water and the importance of water for most biochemical processes.
• What pH is, and why maintaining a proper pH in the body is important for biochemical reactions.
Why do we need to study chemistry? Everything on Earth is ruled by the laws of chemistry. Chemistry is the study of matter and energy in both living and nonliving things. It is the study of how energy causes matter to combine, break apart, and recombine into all substances.
2.2 The Building Blocks of Molecules
At its most fundamental level, life is made up of matter. Matter occupies space and has mass. All matter is composed of elements, substances that cannot be broken down or transformed chemically into other substances. Each element is made of atoms, each with a constant number of protons and unique properties. A total of 118 elements have been defined; however, only 92 occur naturally, and fewer than 30 are found in living cells. The remaining 26 elements are unstable and, therefore, do not exist for very long or are theoretical and have yet to be detected. Each element is designated by its chemical symbol (such as H, N, O, C, and Na), and possesses unique properties. These unique properties allow elements to combine and to bond with each other in specific ways. The periodic table of the elements arranges elements in groups based on their properties. The element most important to life is carbon (C).
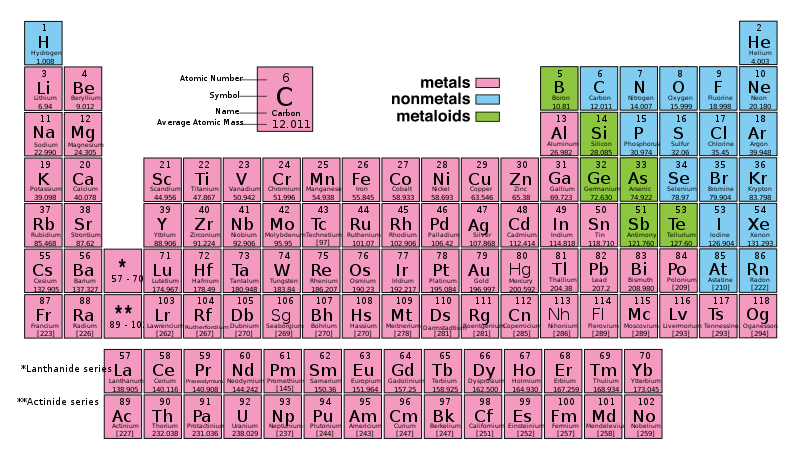
Figure 2.1: Periodic Table of the Elements. The periodic table of the elements arranges elements in groups based on their properties. The periodic table provides key information about the elements and how they might interact with each other to form molecules. Most periodic tables provide a key or legend to the information they contain.
Atoms
An atom is the smallest particle of an element that still has the properties of that element. Every substance is composed of atoms. Atoms are extremely small, typically about a ten-billionth of a meter in diameter. All atoms contain subatomic particles; protons, electrons, and neutrons (Figure 2.2). The only exception is hydrogen (H), which is made of one proton and one electron.
A proton is a positively charged particle that resides in the nucleus (the core of the atom) of an atom and has a mass of 1 and a charge of +1. An electron is a negatively charged particle that travels in the space around the nucleus. In other words, it resides outside of the nucleus. It has a negligible mass (i.e. considered to be zero compared to protons and neutrons) and has a charge of -1. Neutrons, like protons, reside in the nucleus of an atom. They have a mass of 1 and no charge. The positive (protons) and negative (electrons) charges balance each other in a neutral atom, which has a net zero charge.
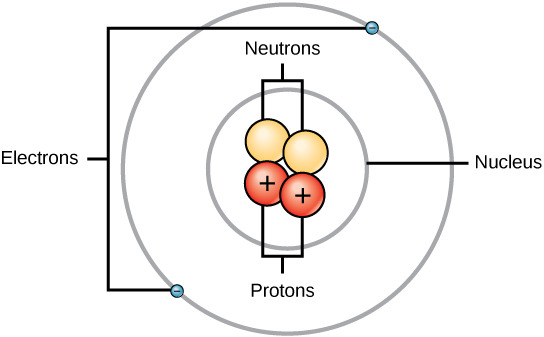
Figure 2.2: Atoms are made up of protons and neutrons located within the nucleus, and electrons surrounding the nucleus
Because protons and neutrons each have a mass of 1, the mass of an atom is equal to the combined number of protons and neutrons of that atom. The number of electrons does not factor into the overall mass, because their mass is so small. As stated earlier, each element has its own unique properties. Each contains a different number of protons and neutrons, giving it its own atomic number and mass number. The atomic number of an element is equal to the number of protons that element contains. The mass number, or atomic mass, is the number of protons plus the number of neutrons of that element. Therefore, it is possible to determine the number of neutrons by subtracting the atomic number from the mass number. For example, the element phosphorus (P) has an atomic number of 15 and a mass number of 31. Therefore, an atom of phosphorus has 15 protons, 15 electrons, and 16 neutrons (31-15 = 16).
These numbers provide information about the elements and how they will react when combined. Each element has different characteristics and properties. Because of these characteristics, the elements are arranged into the periodic table of elements, a chart of the elements that includes the atomic number and relative atomic mass of each element. The periodic table also provides key information about the properties of elements (Figure 2.1) often indicated by color-coding. The arrangement of the table also shows how the electrons in each element are organized and provides important details about how atoms will react with each other to form molecules.
Isotopes are different forms of the same element that have the same number of protons, but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes. Carbon-12, the most common isotope of carbon, contains six protons and six neutrons. Therefore, it has a mass number of 12 (six protons and six neutrons) and an atomic number of 6 (which makes it carbon). Carbon-14 contains six protons and eight neutrons. Therefore, it has a mass number of 14 (six protons and eight neutrons) and an atomic number of 6, meaning it is still the element carbon. These two alternate forms of carbon are isotopes. Some isotopes are unstable and will lose protons, other subatomic particles, or energy to form more stable elements. These are called radioactive isotopes or radioisotopes. Carbon-14 (14C) is a naturally occurring radioisotope that is created in the atmosphere by cosmic rays. It is used to age formerly living objects, such as fossils. Through the use of carbon dating, scientists can reconstruct the ecology and biogeography of organisms living within the past 50,000 years
Compounds and Molecules
A compound is a unique substance that consists of two or more elements combined in fixed proportions. This means that the composition of a compound is always the same. The smallest particle of most compounds in living things is called a molecule. Consider water as an example. A molecule of water always contains one atom of oxygen and two atoms of hydrogen. The composition of water is expressed by the chemical formula H2O. A model of a water molecule is shown in Figure 2.3. Notice that molecules can be drawn in different ways but represent the same molecule. In this case, a molecule made of one oxygen and two hydrogens.
What causes the atoms of a water molecule to “stick” together? The answer is chemical bonds. A chemical bond is a force that holds together the atoms of molecules. Bonds in molecules involve atoms sharing electrons. New chemical bonds form when substances react with one another.
Energy
Joining atoms together to form molecules and breaking apart molecules requires energy. Energy is defined as the capacity to do work. When an object is in motion, there is energy associated with that object. Think of a wrecking ball. Even a slow-moving wrecking ball can do a great deal of damage to other objects. Energy associated with objects in motion is called kinetic energy. A speeding bullet, a walking person, and the rapid movement of molecules in the air (which produces heat) all have kinetic energy. Now what if that same motionless wrecking ball is lifted two stories above ground with a crane? If the suspended wrecking ball is unmoving, is there energy associated with it? The answer is yes. The energy that was required to lift the wrecking ball did not disappear but is now stored in the wrecking ball by virtue of its position and the force of gravity acting on it. This type of energy is called potential energy. If the ball were to fall, the potential energy would be transformed into kinetic energy until all of the potential energy was exhausted when the ball rested on the ground. Wrecking balls also swing like a pendulum; through the swing, there is a constant change of potential energy (highest at the top of the swing) to kinetic energy (highest at the bottom of the swing). Other examples of potential energy include the energy of water held behind a dam or a person about to skydive out of an airplane
Explore More
Watch the video below to learn more about the size of an atom.
Review
- What is an element? Give three examples.
- Define compound. Explain how compounds form.
- Compare and contrast atoms and molecules.
- The compound called water can be broken down into its constituent elements by applying an electric current to it. What ratio of elements is produced in this process?
- Relate ions to elements and atoms.
- What is the most important element of life?
- Explain why ions have a positive or negative charge.
- Name the three subatomic particles described in this section.
- What is the difference between kinetic energy and potential energy?
2.3: Chemical Bonding
How elements interact with one another depends on how their electrons are arranged and how many openings for electrons exist at the outermost region where electrons are present in an atom. Electrons exist at energy levels that form shells around the nucleus. The closest shell can hold up to two electrons. The closest shell to the nucleus is always filled first, before any other shell can be filled. Hydrogen has one electron; therefore, it has only one spot occupied within the lowest shell. Helium has two electrons; therefore, it can completely fill the lowest shell with its two electrons. If you look at the periodic table, you will see that hydrogen and helium are the only two elements in the first row. This is because they only have electrons in their first shell. Hydrogen and helium are the only two elements that have the lowest shell and no other shells. The second and third energy levels can hold up to eight electrons.
Not all elements have enough electrons to fill their outermost shells, but an atom is most stable when all of the electron positions in the outermost shell are filled. Because of these vacancies in the outermost shells, we see the formation of chemical bonds, or interactions between two or more of the same or different elements that result in the formation of molecules. To achieve greater stability, atoms will tend to completely fill their outer shells and will bond with other elements to accomplish this goal by sharing electrons, accepting electrons from another atom, or donating electrons to another atom. Because the outermost shells of the elements with low atomic numbers (up to calcium, with atomic number 20) can hold eight electrons, this is referred to as the octet rule. An element can donate, accept, or share electrons with other elements to fill its outer shell and satisfy the octet rule.
Not all chemical bonds form in the same way as the bonds in water. There are actually four different types of chemical bonds that we will discuss here are non-polar covalent, polar covalent, hydrogen, and ionic bonding.
Covalent Bonds
A type of chemical bond between two or more atoms is a covalent bond. These bonds form when an electron is shared between two elements and are the strongest and most common form of chemical bond in living organisms. Covalent bonds form between the elements that make up the biological molecules in our cells. Covalent bonds do not dissociate (i.e. separate) in water. The hydrogen and oxygen atoms that combine to form water molecules are bound together by covalent bonds. The electron from the hydrogen atom divides its time between the outer shell of the hydrogen atom and the incomplete outer shell of the oxygen atom. To completely fill the outer shell of an oxygen atom, two electrons from two hydrogen atoms are needed, hence the subscript 2 in H2O. The electrons are shared between the atoms, dividing their time between them to fill the outer shell of each. This sharing is a lower energy state for all of the atoms involved than if they existed without their outer shells filled.
Figure 2.4: Bonds between hydrogen and oxygen atoms in a water molecule. Electrons are drawn as spheres on the circle indicating an electron orbit. The nucleus is shown as the big sphere in the center of the atom.
There are two types of covalent bonds: polar and nonpolar.
Non-polar Covalent Bonds
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally. For example, an oxygen atom can bond with another oxygen atom to fill their outer shells. This association is nonpolar because the electrons will be equally distributed between each oxygen atom. Two covalent bonds form between the two oxygen atoms because oxygen requires two shared electrons to fill its outermost shell. Nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three electrons to fill its outermost shell. Another example of a nonpolar covalent bond is found in the methane (CH4) molecule. The carbon atom has four electrons in its outermost shell and needs four more to fill it. It gets these four from four hydrogen atoms, each atom providing one. These elements all share the electrons equally, creating four nonpolar covalent bonds (Figure 2.5).
Figure 2 5 Methane is formed when four hydrogens and one carbon covalently bond.
Polar Covalent Bonds
In a polar covalent bond, the electrons shared by the atoms spend more time closer to one nucleus than to the other nucleus. Because of the unequal distribution of electrons between the different nuclei, a slightly positive (+) or slightly negative (-) charge develops. The covalent bonds between hydrogen and oxygen atoms in water are polar covalent bonds. The shared electrons spend more time near the oxygen nucleus, giving it a small negative charge, than they spend near the hydrogen nuclei, giving these molecules a small positive charge.
The water molecule represented in Figure 2.6 contains polar covalent bonds. The hydrogen atoms are bound to the highly electronegative oxygen atom which also possesses two lone pair sets of electrons, making for a very polar bond. The partially positive hydrogen atom of one molecule is then attracted to the partially negative oxygen atom of a nearby water molecule as shown in Figure 2.6.
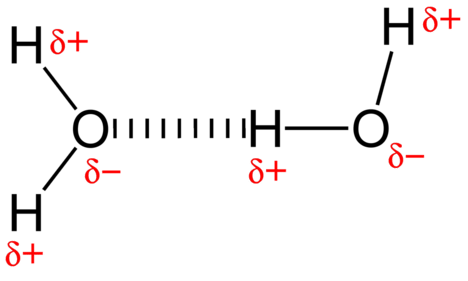
Figure 2.6: A hydrogen bond in water occurs between the hydrogen atom of one water molecule and the lone pair of electrons on an oxygen atom of a neighboring water molecule.
Ionic bonds
Electrons are transferred between atoms. Some atoms may have only one or two electrons in their outermost shells while others need one or two to fill their outermost shells, Atoms that need an electron or two will accept electrons from another atom that only has one or two electrons in its outermost shell. When an atom does not contain equal numbers of protons and electrons, it is called an ion. Because the number of electrons does not equal the number of protons, each ion has a net charge.
For example, sodium only has one electron in its outermost shell. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill the outer shell. If sodium loses an electron, it now has 11 protons and only 10 electrons, leaving it with an overall charge of +1. It is now called a sodium ion. The chlorine atom has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons and 18 electrons, giving it a net negative (-1) charge. It is now called a chloride ion. This movement of electrons from one element to another is referred to as electron transfer. As Figure 2.7 illustrates, a sodium atom (Na) only has one electron in its outermost shell, whereas a chlorine atom (Cl) has seven electrons in its outermost shell. A sodium atom will donate its one electron to empty its shell, and a chlorine atom will accept that electron to fill its shell, becoming chloride. Both ions now satisfy the octet rule and have complete outermost shells. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium) or -1 (chloride) charge
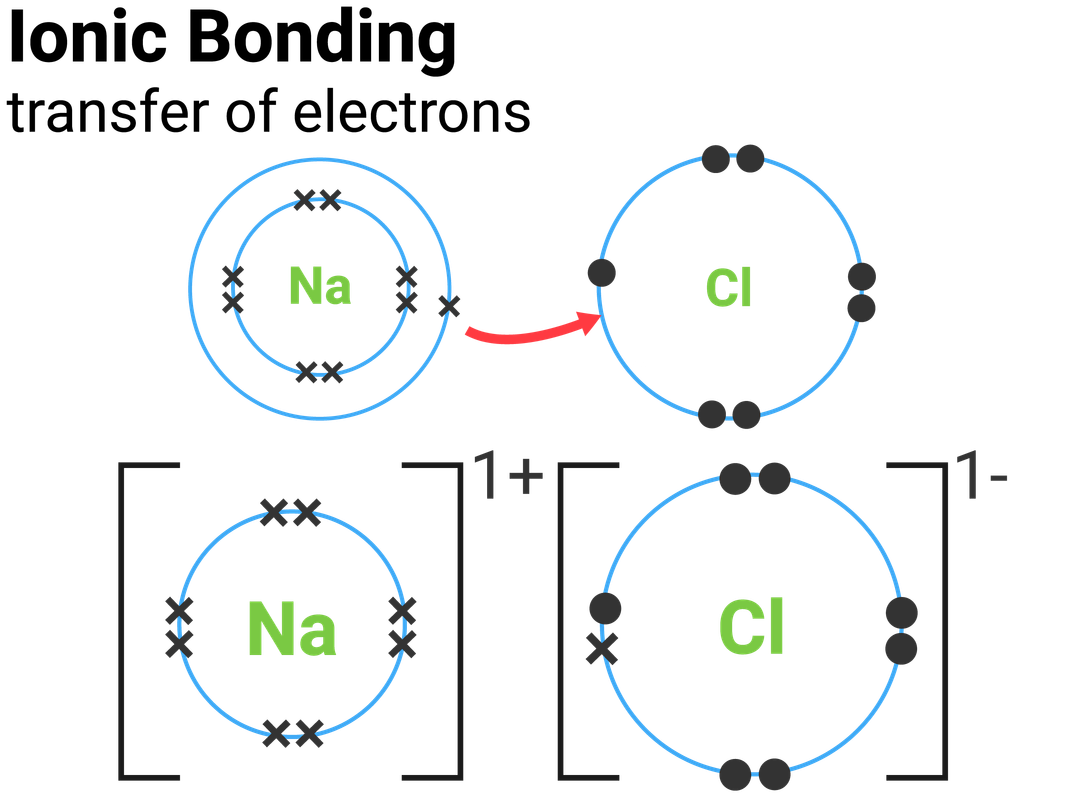
Figure 2.7: Elements tend to ll their outermost shells with electrons. To do this, they can either donate or accept electrons from other elements.
When an element donates an electron from its outer shell, as in the sodium atom example above, a positive ion is formed. The element accepting the electron is now negatively charged. Because positive and negative charges attract, these ions stay together and form an ionic bond, or a bond between ions. The elements bond together with the electron from one element staying predominantly with the other element. When Na+ and Cl ions combine to produce NaCl, an electron from a sodium atom stays with the other seven from the chlorine atom, and the sodium and chloride ions attract each other in a lattice of ions with a net zero charge.
Hydrogen Bonds
Ionic and covalent bonds are strong bonds that require considerable energy to break. However, not all bonds between elements are ionic or covalent bonds. Weaker bonds can also form. These are attractions that occur between positive and negative charges that do not require much energy to break. An example of relatively weak bonds that occur frequently is hydrogen bonds. This bond gives rise to the unique properties of water and the unique structures of DNA and proteins.
When polar covalent bonds containing a hydrogen atom form, the hydrogen atom in that bond has a slightly positive charge. This is because the shared electron is pulled more strongly toward the other element and away from the hydrogen nucleus. Because the hydrogen atom is slightly positive (+), it will be attracted to neighboring negative partial charges (-). When this happens, a weak interaction occurs between the + charge of the hydrogen atom of one molecule and the – charge of the other molecule. This interaction is called a hydrogen bond. This type of bond is common; for example, the liquid nature of water is caused by the hydrogen bonds between water molecules (Figure 2.6). Hydrogen bonds give water the unique properties that sustain life. If it were not for hydrogen bonding, water would be a gas rather than a liquid at room temperature.
Hydrogen bonds can form between different molecules, and they do not always have to include a water molecule. Hydrogen atoms in polar bonds within any molecule can form bonds with other adjacent molecules. For example, hydrogen bonds hold together two long strands of DNA to give the DNA molecule its characteristic double-helix structure. Hydrogen bonds are also responsible for some of the three-dimensional structure of proteins.
Review
- How is a covalent bond different from an ionic bond?
- Why is a hydrogen bond a relatively weak bond?
- Diagram the polarity of a water molecule.
- What is a chemical bond?
- Explain why hydrogen and oxygen atoms are more stable when they form bonds in a water molecule.
- How many valence electrons does sodium have? How many valence electrons does chlorine have? How does a chlorine atom bonds with sodium? What is the charge on a sodium ion? What about the chlorine ion?
- When does covalent bonding occur? How does it work?
- How many valence electrons does oxygen have?
2.4 The Chemical and Physical Properties of Water
Do you ever wonder why scientists spend time looking for water on other planets? It is because water is essential to life; even minute traces of it on another planet can indicate that life could or did exist on that planet. Water is one of the more abundant molecules in living cells and the one most critical to life as we know it. Approximately 60-70 percent of your body is made up of water. Without it, life simply would not exist.
Water Is Polar
The hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. Because of these charges, the slightly positive hydrogen atoms repel each other. Each water molecule attracts other water molecules because of the positive and negative charges in the different parts of the molecule. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with water, it can dissolve in water and is referred to as hydrophilic (water-loving). Hydrogen bonds are not readily formed with nonpolar substances like oils and fats. These nonpolar compounds are hydrophobic (water-fearing) and will not dissolve in water.
Water Stabilizes Temperature
The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances. Temperature is a measure of the motion (kinetic energy) of molecules. As the motion increases, energy is higher and thus temperature is higher. Water absorbs a great deal of energy before its temperature rises. Increased energy disrupts the hydrogen bonds between water molecules. Because these bonds can be created and disrupted rapidly, water absorbs an increase in energy and temperature changes only minimally. This means that water moderates temperature changes within organisms and in their environments. As energy input continues, the balance between hydrogen-bond formation and destruction swings toward the destruction side. More bonds are broken than are formed. This process results in the release of individual water molecules at the surface of the liquid (such as a body of water, the leaves of a plant, or the skin of an organism) in a process called evaporation. Evaporation of sweat, which is 90 percent water, allows for cooling of an organism, because breaking hydrogen bonds requires an input of energy and takes heat away from the body.
Water Is an Excellent Solvent
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent – a substance capable of dissolving another substance, referred to as the solute, in order to form a solution. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. The particles will remain separated or dispersed in the water. In the case of table salt (NaCl) mixed in water (Figure 2.8), the sodium and chloride ions separate, or dissociate in the water. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
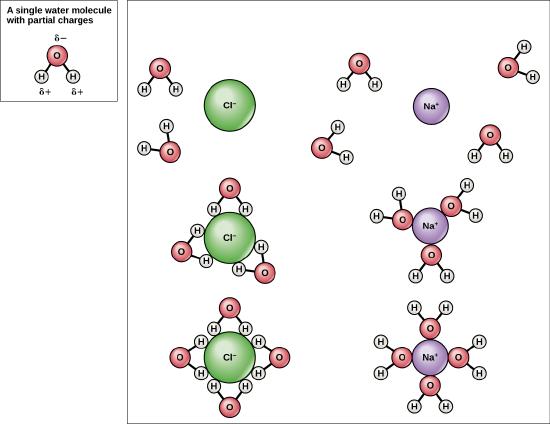
Figure 2.8: This diagram shows the positive and negative parts of a water molecule. It also depicts how a charge, such as on an ion (Na or Cl, for example) can interact with a water molecule.
Review
- Describe the structure of a water molecule. What is polarity, and why is water polar?
- Explain how the internal polarity of the water molecule makes it a good solvent?
- What property of water helps to maintain homeostasis and how?
2.5 Buffers, pH, Acids, and Bases
The pH of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper, paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, to test how much acid or base (alkalinity) exists in a solution. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exists in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 2.9). Therefore, the more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH. The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic and has a pH of 7.0. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0).
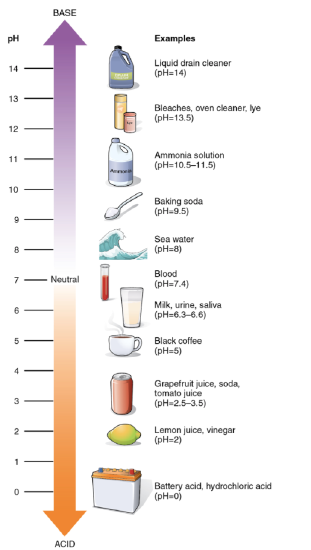
Figure 2.9: The pH scale measures the amount of hydrogen ions (H+) in a substance.
Acids are substances that provide hydrogen ions (H+) and lower pH, whereas bases provide hydroxide ions (OH) and raise pH. The stronger the acid, the more readily it donates H+. For example, hydrochloric acid and lemon juice are very acidic and readily give up H+ when added to water. Conversely, bases are those substances that readily donate OH. The OH ions combine with H+ to produce water, which raises a substance’s pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH rapidly when placed in water, thereby raising the pH.
Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. Cells no longer function properly, and proteins will break down. Deviation outside of the pH range can induce coma or even cause death.
So how is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H+ or OH, keeping the pH of the body carefully maintained in the aforementioned narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it keeps the pH within the proper range. This buffer system involves carbonic acid (H2CO3) and bicarbonate (HCO3 ) anion. If too much H+ enters the body, bicarbonate will combine with the H+ to create carbonic acid and limit the decrease in pH. Likewise, if too much OH– is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H+ ions. The H+ ions can combine with the OH– ions, limiting the increase in pH. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Figure 2.10: The bicarbonate buffer system.
2.6 Biological Macromolecules
The large molecules necessary for life that are built from smaller organic molecules are called biological macromolecules. There are four major classes of biological macromolecules (carbohydrates, lipids, proteins, and nucleic acids), and each is an important component of the cell and performs a wide array of functions. Combined, these molecules make up the majority of a cell’s mass. Biological macromolecules are organic, meaning that they contain carbon. In addition, they may contain hydrogen, oxygen, nitrogen, phosphorus, sulfur, and additional minor elements.
Carbon
It is often said that life is carbon-based. This means that carbon atoms, bonded to other carbon atoms or other elements, form the fundamental components of many, if not most, of the molecules found uniquely in living things. Other elements play important roles in biological molecules, but carbon certainly qualifies as the foundation element for molecules in living things. It is the bonding properties of carbon atoms that are responsible for its important role.
Carbon contains four electrons in its outer shell. Therefore, it can form four covalent bonds with other atoms or molecules. The simplest organic carbon molecule is methane (CH4), in which four hydrogen atoms bind to a carbon atom (Figure 2.5).
However, structures that are more complex are made using carbon. Any of the hydrogen atoms can be replaced with another carbon atom covalently bonded to the first carbon atom. In this way, long and branching chains of carbon compounds can be made. The carbon atoms may bond with atoms of other elements, such as nitrogen, oxygen, and phosphorus. The molecules may also form rings, which themselves can link with other rings. This diversity of molecular forms accounts for the diversity of functions of the biological macromolecules and is based to a large degree on the ability of carbon to form multiple bonds with itself and other atoms.
Making and Breaking Biological Macromolecules
Dehydration Synthesis
Most macromolecules are made from single subunits, or building blocks, called monomers. The monomers combine with each other using covalent bonds to form larger molecules known as polymers. In doing so, monomers release water molecules as byproducts. This type of reaction is known as dehydration synthesis, which means “to put together while losing water.” You will sometimes see these types of building reactions referred to as simply dehydration reactions or synthesis reactions or even as condensation reactions because a molecule of water is produced as a result.

In a dehydration synthesis reaction (Figure 2.11), the hydrogen of one monomer combines with the hydroxyl group of another monomer, releasing a molecule of water. At the same time, the monomers share electrons and form covalent bonds. As additional monomers join, this chain of repeating monomers forms a polymer. Different types of monomers can combine in many configurations, giving rise to a diverse group of macromolecules. Even one kind of monomer can combine in a variety of ways to form several different polymers: for example, glucose monomers are the constituents of starch, glycogen, and cellulose.
Hydrolysis
Polymers are broken down into monomers in a process known as hydrolysis, which means “to split water,” a reaction in which a water molecule is used during the breakdown (Figure 2.12). During these reactions, the polymer is broken into two components: one part gains a hydrogen atom (H+) and the other gains a hydroxyl molecule (OH–) from a split water molecule.

Dehydration and hydrolysis reactions are catalyzed, or “sped up,” by specific enzymes; dehydration reactions involve the formation of new bonds, requiring energy, while hydrolysis reactions break bonds and release energy. These reactions are similar for most macromolecules, but each monomer and polymer reaction is specific for its class. For example, in our bodies, food is hydrolyzed, or broken down, into smaller molecules by catalytic enzymes in the digestive system. This allows for easy absorption of nutrients by cells in the intestine. Each macromolecule is broken down by a specific enzyme. For instance, carbohydrates are broken down by amylase, sucrase, lactase, or maltase.
Review
- What is the difference between dehydration and hydrolysis reactions?
- What are the four classes of macromolecules?
- What is a monomer? A polymer?
- Macromolecules have a backbone of ____________________ atoms.
Carbohydrates
Carbohydrates are the most common class of biochemical compounds. They include sugars and starches. Carbohydrates are used to provide or store energy, among other uses. Like most biochemical compounds, carbohydrates are built of small repeating units, or monomers, which form bonds with each other to make larger molecules, called polymers. In the case of carbohydrates, the small repeating units are known as monosaccharides. Each monosaccharide consists of six carbon atoms, as shown in the model of the monosaccharide glucose below.
Sugars are the general name for sweet, short-chain, soluble carbohydrates, which are found in many foods. Their function in living things is to provide energy. The simplest sugars consist of a single monosaccharide. They include glucose, fructose, and galactose. Glucose is a simple sugar that is used for energy by the cells of living things. Fructose is a simple sugar found in fruits, and galactose is a simple sugar found in milk.
Other sugars contain two monosaccharide molecules and are called disaccharides. An example is sucrose or table sugar. It is composed of one fructose molecule and one glucose molecule. Other disaccharides include maltose (two glucose molecules) and lactose (one glucose molecule and one galactose molecule). Lactose occurs naturally in milk. Some people can’t digest lactose. If they drink milk, it causes gas, cramps, and other unpleasant symptoms unless the milk has been processed to remove the lactose.
Glucose and fructose combine to produce the disaccharide sucrose in a dehydration reaction as shown in Figure 2.14. Sucrose, commonly known as table sugar, is an example of a disaccharide.
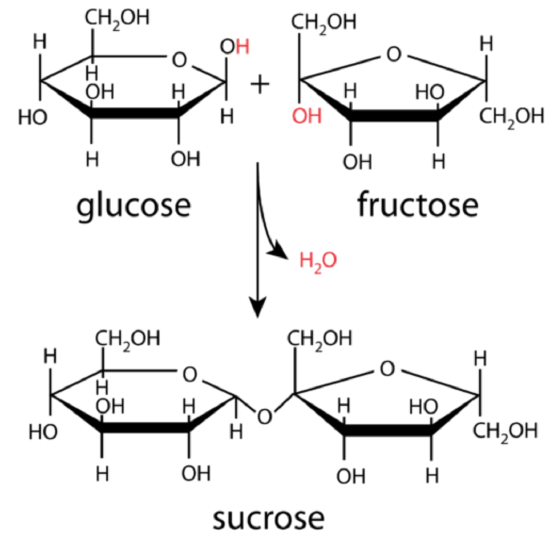
Figure 2.14: Glucose and fructose combine to produce the disaccharide sucrose in a condensation reaction. The diagram shows how water is produced when the reaction occurs. This is because the oxygen in glucose binds to the carbon in fructose. That removes an oxygen and two hydrogens from the new molecule.
Some carbohydrates consist of hundreds or even thousands of monosaccharides bonded together in long chains. These carbohydrates are called polysaccharides (“many saccharides”). Polysaccharides are also referred to as complex carbohydrates. Complex carbohydrates that are found in living things include starch, glycogen, cellulose, and chitin. Each type of complex carbohydrate has different functions in living organisms but they generally either store energy or make up certain structures of living things.
Review
- What are carbohydrates? Describe their structure.
- Compare and contrast sugars and complex carbohydrates.
- If you chew on a starchy food such as a saltine cracker for several minutes, it may start to taste sweet. Explain why.
- Put the following carbohydrates in order from smallest to largest: cellulose; fructose; sucrose
- Name three carbohydrates that contain glucose as a monomer.
- Jeans are made of tough, durable cotton. Explain how you think this fabric gets its tough qualities, based on what you know about the structure of carbohydrates.
- Which do you think is faster to digest — simple sugars or complex carbohydrates? Explain your answer.
- True or False. Cellulose is broken down in the human digestive system into glucose molecules.
- Which carbohydrate is used directly by the cells of living things for energy.
Explore More
Watch the video below to learn about the health impacts of carbohydrates.
Lipids
Fats are actually a type of lipid. Lipids are a major class of biochemical compounds that includes oils as well as fats. Organisms use lipids to store energy and for many other uses. A fat molecule, such as a triglyceride, consists of two main components glycerol and fatty acids. Glycerol is an organic compound with three carbon atoms, five hydrogen atoms, and three hydroxyl (OH) groups. Fatty acids have a long chain of hydrocarbons to which an acidic carboxyl group (-COOH) is attached, hence the name fatty acid. The number of carbons in the fatty acid may range from 4 to 36; most common are those containing 1218 carbons. In a fat molecule, a fatty acid is attached to each of the three oxygen atoms in the OH groups of the glycerol molecule with a covalent bond (Figure 2.16) created by dehydration-synthesis reactions.
There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids. Both types consist mainly of simple chains of carbon atoms bonded to one another and to hydrogen atoms. The two types of fatty acids differ in how many hydrogen atoms they contain. In saturated fatty acids, carbon atoms are bonded to as many hydrogen atoms as possible. All the carbon-to-carbon atoms share just single bonds between them. This causes the molecules to form straight chains, as shown in Figure 2.15. The straight chains can be packed together very tightly, allowing them to store energy in a compact form. Saturated fatty acids have relatively high melting points, explaining why they are solids at room temperature. Animals use saturated fatty acids to store energy.
In unsaturated fatty acids, some carbon atoms are not bonded to as many hydrogen atoms as possible. Instead, they form double or even triple bonds with other carbon atoms. This causes the chains to bend (see Figure 2.15). The bent chains cannot be packed together very tightly. Unsaturated fatty acids have relatively low melting points, which explains why they are liquids at room temperature. Plants use unsaturated fatty acids to store energy.
Monounsaturated fatty acids contain one less hydrogen atom than the same-length saturated fatty acid chain. Monounsaturated fatty acids are liquids at room temperature but start to solidify at refrigerator temperatures. Good food sources of monounsaturated fats include olive and peanut oils and avocados.
Polyunsaturated fatty acids contain at least two fewer hydrogen atoms than the same-length saturated fatty acid chain. Polyunsaturated fatty acids are liquids at room temperature and remain in the liquid state in the refrigerator. Good food sources of polyunsaturated fats include safflower and soybean oils and many nuts and seeds.
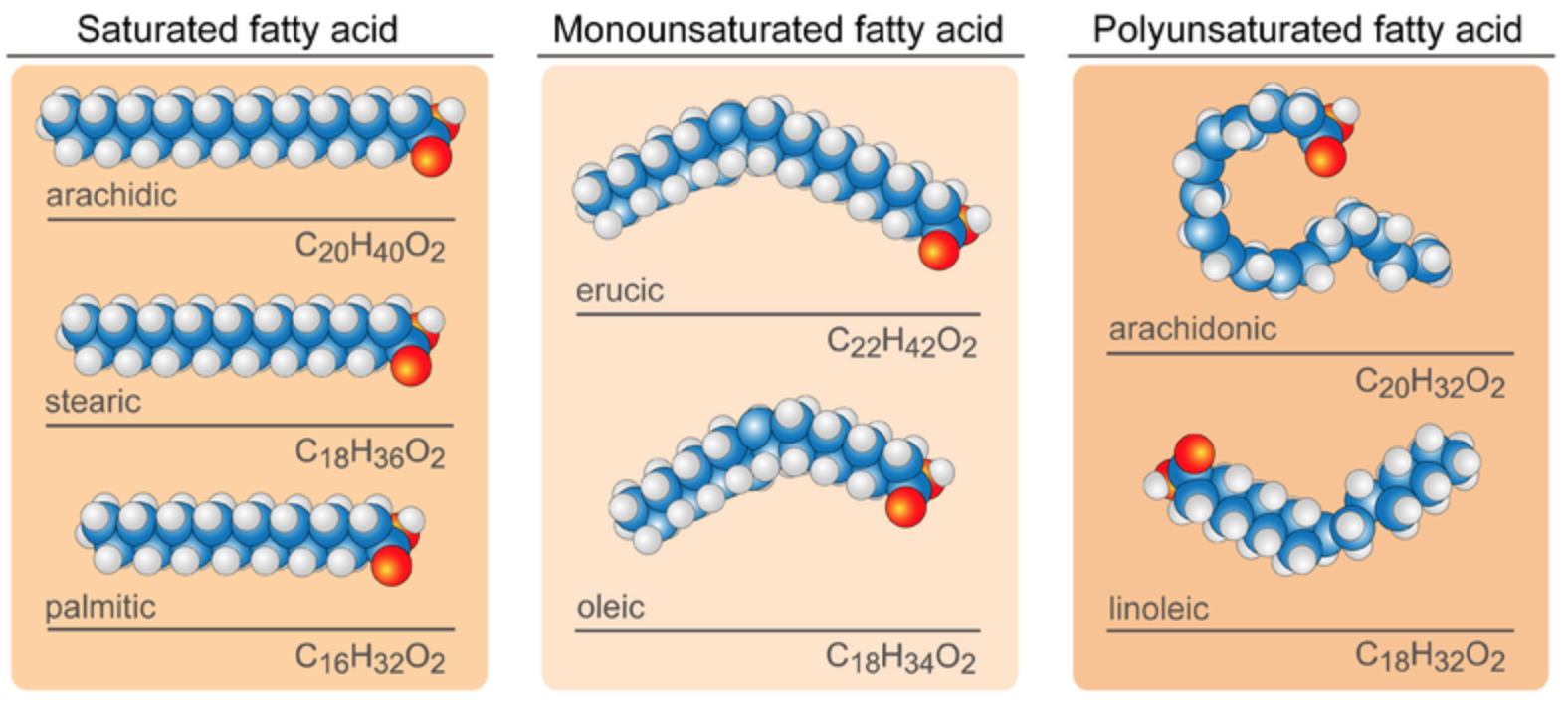
Figure 2.15: Fatty Acids models. The blue balls, white balls, and red balls represent carbon, hydrogen, and oxygen, respectively. Saturated fatty acids, such as arachidic, stearic and palmitic, have straight chains. Unsaturated fatty acids have bent chains. Monounsaturated fatty acids, such as erucic and oleic have a single double bond between carbons creating a single bend in the chain. Polyunsaturated fatty acids, such as arachidoic and linoleic, have multiple carbon-carbon double bonds creating multiple bends.
Types of lipids include triglycerides, phospholipids, and steroids. Each type has different functions in living things.
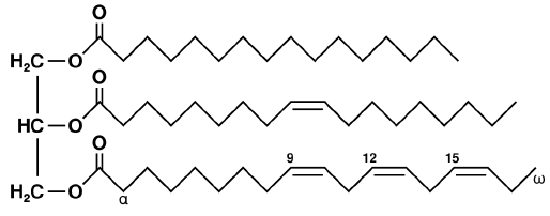
Triglycerides are formed by combining a molecule of glycerol with three fatty acid molecules. Glycerol (also called glycerine) is a simple compound known as a sugar alcohol. It is a colorless, odorless liquid that is sweet tasting and nontoxic. Triglycerides are the main constituent of body fat in humans and other animals. They are also found in fats derived from plants. There are many different types of triglycerides, with the main division being between those that contain saturated fatty acids and those that contain unsaturated fatty acids.
In the human bloodstream, triglycerides play an important role in metabolism as energy sources and transporters of dietary fat. They contain more than twice as much energy as carbohydrates, the other major source of energy in the diet. When you eat, your body converts any calories it doesn’t need to use right away into triglycerides, which are stored in your fat cells. When you need energy between meals, hormones trigger the release of some of these stored triglycerides back into the bloodstream.
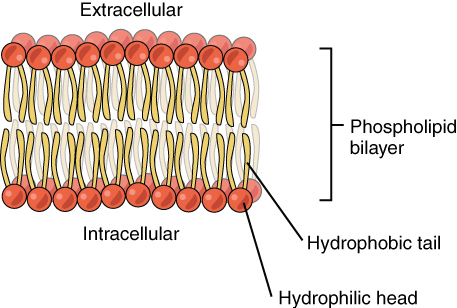
Phospholipids are a major component of the cell membranes of all living things. Each phospholipid molecule has a “tail” consisting of two long fatty acids and a “head” consisting of a phosphate group and glycerol molecule (see diagram below). The phosphate group is a small negatively charged molecule. The phospholipid head is hydrophilic or attracted to water. The fatty acid tail of the phospholipid is hydrophobic or repelled by water. These properties allow phospholipids to form a two-layer, or bilayer, cell membrane.
As shown in the diagram below, a phospholipid bilayer forms when many phospholipid molecules line up tail to tail, forming an inner and outer surface of hydrophilic heads. The hydrophilic heads point toward both the watery extracellular space and the watery intracellular space (lumen) of the cell.
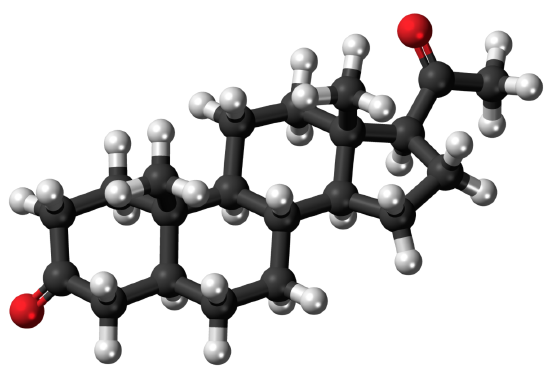
Steroids are lipids with a ring structure. Each steroid has a core of seventeen carbon atoms arranged in four rings of five or six carbons each (see model pictured below). Steroids vary by the other components attached to this four-ring core. Hundreds of steroids are found in plants, animals, and fungi, but most steroids have one of just two principal biological functions: some steroids, such as cholesterol, are important components of cell membranes; many other steroids are hormones, which are messenger molecules. In humans, steroid hormones include cortisone, a fight-or-flight hormone; and the sex hormones estrogen and testosterone.
Review
- What are lipids?
- Compare and contrast saturated and unsaturated fatty acids.
- Identify three major types of lipids, and describe differences in their structures.
- How do triglycerides play an important role in human metabolism?
- Explain how phospholipids form cell membranes.
- What is cholesterol, and what is its major function?
- Give three examples of steroid hormones in humans.
- Which type of fatty acid do you think is predominant in the steak and cheese of the cheesesteak shown above? Explain your answer.
- Which type of fat would be the most likely to stay liquid in colder temperatures — bacon fat, olive oil, or soybean oil? Explain your answer.
- Why do you think that the shape of the different types of fatty acid molecules affects how easily they solidify?
Explore More
Watch the video below to learn more about triglycerides and the difference between saturated and unsaturated fatty acids.
Proteins
Proteins are organic compounds that contain carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur. These compounds have many essential functions within the cell (see below). Proteins are made of smaller units called amino acids. There are 20 different common amino acids needed to make proteins. All amino acids have the same basic structure, which is shown in Figure 2.19. Only the side chain (labeled R in the figure) differs from one amino acid to another. These side chains can vary in size from just one hydrogen atom in glycine to a large heterocyclic group in tryptophan. The variable side chain gives each amino acid unique properties. The side chains can also characterize the amino acid as (1) nonpolar or hydrophobic, (2) neutral (uncharged) but polar, (3) acidic, with a net negative charge, and (4) basic, with a net positive charge at neutral pH.

Figure 2.19: General Structure of Amino Acids. This model shows the general structure of all amino acids. Only the side chain, R, varies from one amino acid to another. For example, in the amino acid glycine, the side chain is simply hydrogen (H). In glutamic acid, in contrast, the side chain is
Proteins can differ from one another in the number and sequence (order) of amino acids. It is because of the side chains of the amino acids that proteins with different amino acid sequences have different shapes and different chemical properties. Small proteins can contain just a few hundred amino acids. Yeast proteins average 466 amino acids. The largest known proteins are the titins, found in muscle, which are composed of over 27,000 amino acids.
Protein Structure
Amino acids join together to form a molecule called a dipeptide. The –OH from the carboxyl group of one amino acid combines with a hydrogen atom from the amino group of the other amino acid to produce water. This is dehydration synthesis (condensation reaction) – a reaction in which two molecules combine to form a single molecule with a release of water. Figure 2.20 shows this process. The top part of the image shows two amino acids; note the -OH in amino acid 1 and the -H in amino acid two are highlighted. These are the atoms that will be removed from the amino acids to form water. This allows a covalent bond forms between the carboxyl carbon of one amino acid and the amine nitrogen of the second amino acid. This reaction forms a molecule called a dipeptide and the carbon-nitrogen covalent bond is called a peptide bond. When repeated numerous times, a lengthy molecule called a polypeptide is eventually produced. Very lengthy polypeptides with functional configuration are called proteins.
Figure 2.20: Amino acids join together to form a molecule called a dipeptide. The C−N bond is called a peptide bond. The order of amino acids is by convention shown with the free amino group on the left and the free carboxyl group on the right.
Proteins may have up to four levels of structure, from primary to quaternary, as described and shown in the diagram below, giving them the potential for tremendous diversity:
- A protein’s primary structure is the sequence of amino acids in its polypeptide chain(s). This sequence of amino acids determines the higher levels of protein structure and is encoded in genes.
- A protein’s secondary structure consists of regularly repeating local structures stabilized by hydrogen bonding between the carboxylic and amino groups of the backbone. The most common secondary structures include the alpha-helix and beta-sheet. Because secondary structures are local, many regions of different secondary structures can be present in the same protein molecule.
- A protein’s tertiary structure refers to the overall three-dimensional shape of a single protein molecule. It is determined by the spatial relationship of non-covalent and covalent bonds between the “R” groups of distant amino acids in a polypeptide. The tertiary structure is what controls the basic function of the protein.
- Not all proteins have a final, quaternary structure. This is a structure formed by several protein molecules that function together as a single protein complex.

Figure 2.21: The levels of structure of a protein called hemoglobin.
Functions of Proteins
The diversity of protein structures explains how this class of biochemical compounds can play so many important roles in living things. What are the roles of proteins?
- Some proteins have structural functions. They may help cells keep their shape or make up muscle tissues.
- Many proteins are enzymes that speed up chemical reactions in cells. Enzymes are usually highly specific and accelerate only one or a few chemical reactions. Thousands of different biochemical reactions are known to be catalyzed by enzymes, including most of the reactions involved in metabolism. A reaction without an enzyme might take millions of years to complete, whereas, with the proper enzyme, it may take just a few milliseconds!
- Other proteins are antibodies. These are proteins that bind to specific foreign substances, such as proteins on the surface of bacterial cells. This targets the cells for destruction.
- Still, other proteins carry messages or materials. For example, a protein called myoglobin is an oxygen-binding protein found in the muscle tissues of most mammals including humans. You can see a model of the tertiary structure of myoglobin in the figure below.
The chief characteristic of proteins that allows their diverse set of functions is their ability to bind other molecules specifically and tightly. For example, myoglobin can bind specifically and tightly with oxygen.

Figure 2.22: Myoglobin is a protein found in the muscle tissues of most mammals. It binds with oxygen to supply the cells with this element. The model shows the 3D structure of the protein.
A substance that helps a chemical reaction to occur is called a catalyst, and the molecules that catalyze biochemical reactions are called enzymes. Most enzymes are proteins and perform the critical task of lowering the activation energies of chemical reactions inside the cell. Most of the reactions critical to a living cell happen too slowly at normal temperatures to be of any use to the cell. Without enzymes to speed up these reactions, life could not persist. Enzymes do this by binding to the reactant molecules and holding them in such a way as to make the chemical bond-breaking and -forming processes take place more easily. In addition, an enzyme itself is unchanged by the reaction it catalyzes. Once one reaction has been catalyzed, the enzyme is able to participate in other reactions.
The chemical reactants to which an enzyme binds are called the enzyme’s substrates. The location within the enzyme where the substrate binds is called the enzyme’s active site. The active site is where the “action” happens. Since enzymes are proteins, there is a unique combination of amino acid side chains within the active site.
Active sites are subject to influences of the local environment. Increasing the environmental temperature generally increases reaction rates, enzyme catalyzed or otherwise. However, temperatures outside of an optimal range reduce the rate at which an enzyme catalyzes a reaction. Hot temperatures will eventually cause enzymes to denature, an irreversible change in the three-dimensional shape and therefore the function of the enzyme. Enzymes are also suited to function best within a certain pH and salt concentration range, and, as with temperature, extreme pH, and salt concentrations can cause enzymes to denature.

Figure 2.23: In this diagram, a substrate binds the active site of an enzyme and, in the process, both the shape of the enzyme and the shape of the substrate change. The substrate is converted to product, which leaves the active site.
Protein Consumption, Digestion, and Synthesis
Proteins are necessary for the diets of humans and other animals. We cannot make all the different amino acids we need, so we must obtain some of them from the foods we consume. Through the process of digestion, we break down the proteins in food into free amino acids that can then be used to synthesize our own proteins. Protein synthesis from amino acid monomers takes place in all cells and is controlled by genes. Once new proteins are synthesized, they generally do not last very long before they are degraded and their amino acids are recycled. A protein’s lifespan is generally just a day or two in mammalian cells.
Review
- What are proteins?
- How do two amino acids combine together to make a dipeptide?
- Outline the four levels of protein structure.
- Identify four functions of proteins.
- Explain why proteins can take on so many different functions in living things.
- Can you have a protein with both an alpha helix and a beta-sheet? Why or why not?
- Arrange the following in order from the smallest to the largest level of organization: peptide; protein; amino acid; polypeptide.
Nucleic Acids
Nucleic acids are key macromolecules in the continuity of life. They carry the genetic blueprint of a cell and carry instructions for the functioning of the cell. The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is the genetic material found in all living organisms, ranging from single-celled bacteria to multicellular mammals. The other type of nucleic acid, RNA, is mostly involved in protein synthesis. The DNA molecules never leave the nucleus, but instead use an RNA intermediary to communicate with the rest of the cell. Other types of RNA are also involved in protein synthesis and its regulation. DNA and RNA are made up of monomers known as nucleotides. The nucleotides combine with each other to form a polynucleotide, DNA or RNA. Each nucleotide is made up of three components: a nitrogenous base, a pentose (five-carbon) sugar, and a phosphate group (Figure 2.24). Each nitrogenous base in a nucleotide is attached to a sugar molecule, which is attached to a phosphate group. DNA nucleotides contain the sugar deoxyribose and one of the four bases adenine (A), thymine (T), guanine (G), or cytosine (C). RNA nucleotides contain the sugar ribose and one of the four bases A, uracil (U), G, or C, is single stranded and shorter than DNA.
Structure of Nucleic Acids
Each nucleotide consists of three smaller molecules:
- a sugar molecule (the sugar deoxyribose in DNA and the sugar ribose in RNA).
- a phosphate group.
- a nitrogenous base.
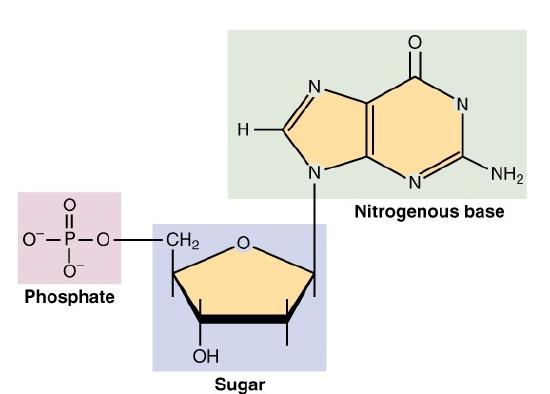
Figure 2.24: Nucleotides consist of a sugar, a nitrogenous base, and a phosphate group
Nucleotides are connected to form DNA as shown in Figure 2.24. The sugar molecule of one nucleotide binds to the phosphate group of the next nucleotide. These two molecules alternate to form the backbone of the nucleotide chain. The nitrogen bases in a nucleic acid stick out from the backbone. There are four different nitrogenous bases: cytosine, adenine, guanine, and either thymine (in DNA) or uracil (in RNA). In DNA, hydrogen bonds form between bases on the two nucleotide chains and hold the chains together. Each type of base binds with just one other type of base: cytosine always bonds with guanine, and adenine always bonds with thymine. These pairs of bases are called complementary base pairs.
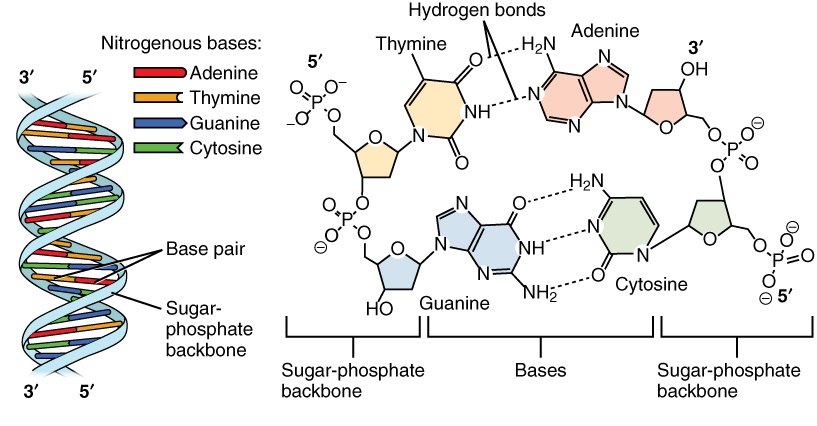
Figure 2.25: Nucleic Acid
The hydrogen bonding of complementary bases causes DNA molecules automatically to take their well-known shape, called a double helix, which is shown in the animation in Figure 2.26.
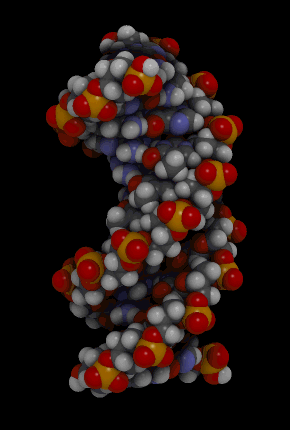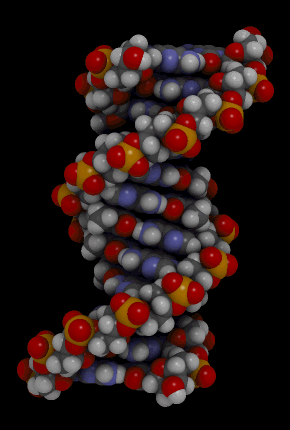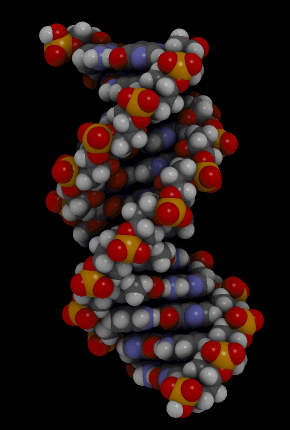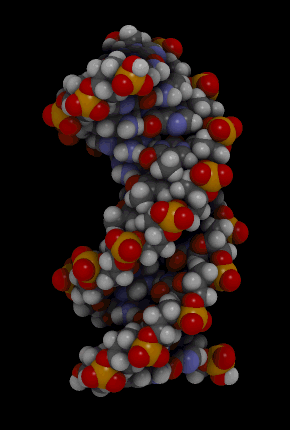
Figure 2.26: DNA molecule
A double helix is like a spiral staircase. The double helix shape forms naturally and is very strong, making the two polynucleotide chains difficult to break apart.
Roles of Nucleic Acids
The DNA of cells is organized into structures called chromosomes as shown in Figure 2.7. The letters A, T, G, and C stand for the bases adenine, thymine, guanine, and cytosine. The sequence of these four bases in DNA is a code that carries instructions for making proteins. The DNA helix is wrapped around proteins called histones to form nucleosomes. These are then further structured into chromatin and, finally, chromosomes. Human cells have 46 chromosomes; other organisms have different numbers of chromosomes.
DNA makes up genes, and the sequence of bases in DNA makes up the genetic code. Between “starts” and “stops,” the code carries instructions for the correct sequence of amino acids in a protein. The information in DNA is passed from parent cells to daughter cells whenever cells divide. The information in DNA is also passed from parents to offspring when organisms reproduce. This is how inherited characteristics are passed from one generation to the next.
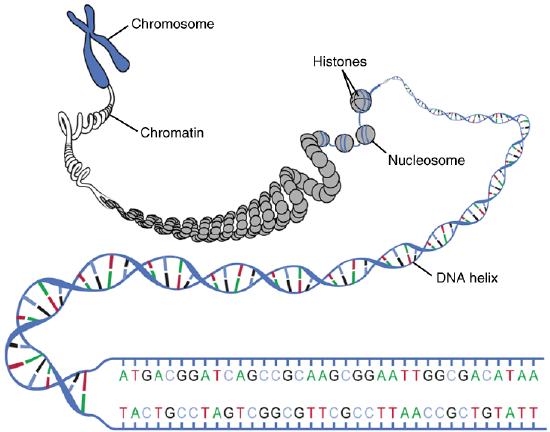
Figure 2.27: The organization of a chromosome
ATP is Energy
There is one type of specialized nucleic acid that exists only as a monomer. It stands apart from the other nucleic acids because it does not code for or help create proteins. This molecule is ATP, which stands for adenosine triphosphate. It consists of a sugar, adenosine, and three phosphate groups. Its primary role is as the basic energy currency in the cell. The way ATP works is all based on the phosphates. As shown in Figure 2.28, a large amount of energy is stored in the bond between the second and third phosphate group. When this bond is broken, energy is released, and this energy can be used to power other processes taking place in the cell.
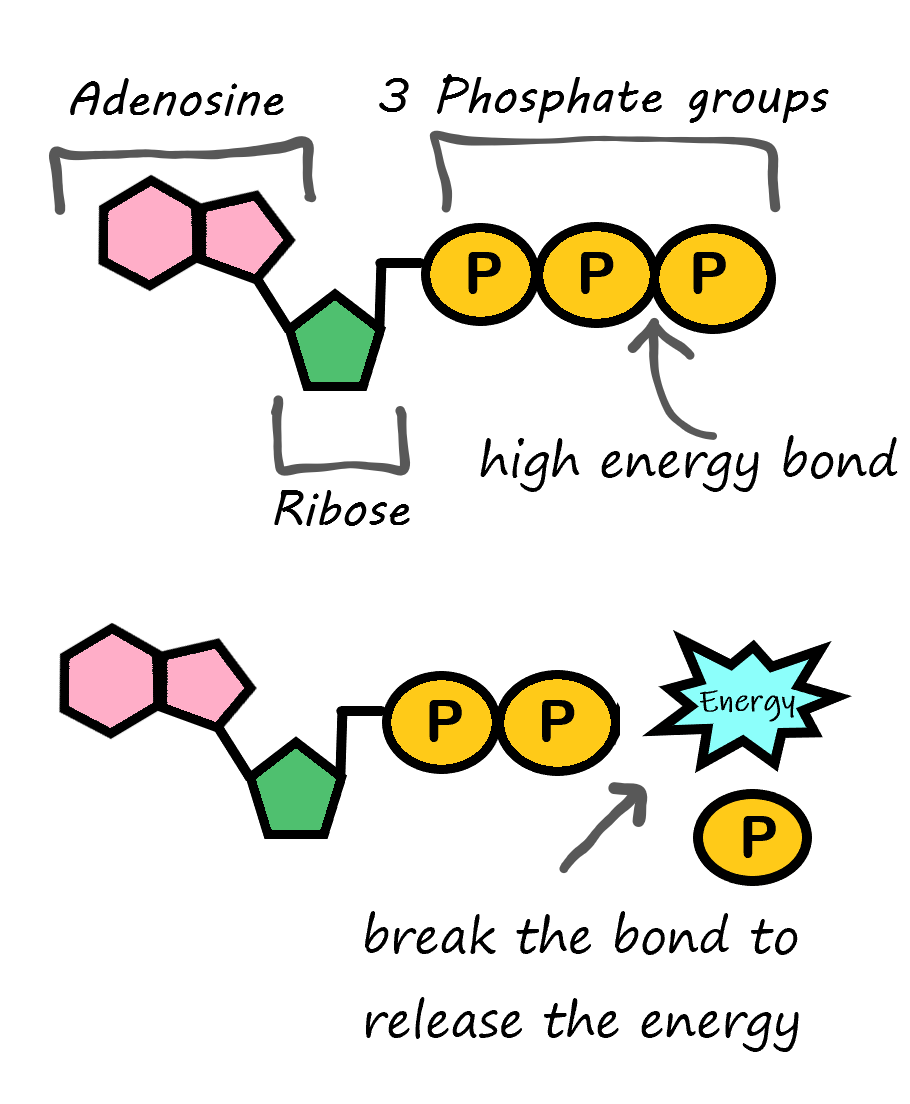
Figure 2.28 ATP (adenosine TRI phosphate) can be converted to ADP (adenosine DI phosphate) to release the energy stored in the chemical bonds between the second and third phosphate group.
Review
- What are the nucleic acids?
- How does RNA differ in structure from DNA?
- Describe a nucleotide. Explain how nucleotides bind together to form a polynucleotide.
- What role do nitrogen bases in nucleotides play in the structure and function of DNA?
- What is the role of RNA?
- True or False. A, C, G, and T represent the bases in RNA.
- True or False. The two polynucleotide chains of RNA twist into a double helix shape.
- True or False. Cytosine always binds to guanine in DNA.
- If part of a chain of DNA has the sequence of bases: ATTG, what is the corresponding sequence of bases that it binds to on the other chain?
- Arrange the following in order from the smallest to the largest level of organization: DNA; nucleotide; polynucleotide
- What is the function of ATP?
- As part of the DNA replication process, the two polynucleotide chains are separated from each other, but each individual chain remains intact. Which bonds are broken in this process?
- Bonds between adjacent sugars and phosphate groups
- Bonds within nucleotides
- Bonds between complementary bases
- Bonds between adenine and guanine
- Adenine, guanine, cytosine, and thymine are:
- Nucleotides
- Nitrogenous bases
- Sugars in DNA and RNA
- Phosphate group
Attributions
This chapter is composed of text taken from of the following sources:
Cushwa, W., & Senior Contributors. (2015). Human biology. OpenStax CNX. [CC BY 4.0 license]. [Retrieved from Human Biology : Willy Cushwa : Free Download, Borrow, and Streaming : Internet Archive]
Gyurkovics, G. (2024). Human biology. CK-12 License. Authored, remixed, and/or curated by S. Wakim & M. Grewal. Edited to the style and standards of the LibreTexts platform. Retrieved from [Human Biology (Gabor Gyurkovics) – Biology LibreTexts]
ISKME. (2024). Biology, the chemistry of life, biological macromolecules, synthesis of biological macromolecules. OER Commons. [License type: CC BY-NC-SA 4.0]. Retrieved from [Biology, The Chemistry of Life, Biological Macromolecules, Synthesis of Biological Macromolecules | OER Commons]
Miller, C. (2020). Human biology: Human anatomy and physiology. Thompson Rivers University. [CC BY NC]. Retrieved from [Human Biology – Simple Book Publishing (tru.ca)]
Postimages. (n.d.). Figure 2.7 ionic bonding image 6 ionic bonding 01 [Image]. Postimages. 6 ionic bonding 01 — Postimages (postlmg.cc)
Search Images. (n.d.). Figure 2.23 enzyme action [Image]. Bing. Mechanism of Enzymes Action – GeeksforGeeks
Search Images. (n.d.). Figure 2.10 blood buffer system [Image]. Bing. Blood Buffer System (easy-saad.blogspot.com)



